Calculate the enthalpy change (in kJ) associated with the conversion of 25.0 grams of ice at -4.00 °C to water vapor at 109.0 °C.The specific heats of ice,water,and steam are 2.09 J/g-K,4.18 J/g-K,and 1.84 J/g-K,respectively.For H2O,Δ 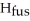 = 6.01 kJ/mol and Δ
= 6.01 kJ/mol and Δ 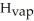 = 40.67 kJ/mol.
= 40.67 kJ/mol.
Definitions:
Selling Price
The amount of money for which a product or service is sold to the buyer.
Subject Matter
The specific topic, area, or thing under discussion or consideration, often within a legal, academic, or artistic context.
Portrait Painter
An artist specialized in creating representations of individuals, typically focusing on their facial features and expressions to convey personality and mood.
Assignment
The conveyance of ownership or entitlements between two parties.
Q3: The vapor pressure of pure water at
Q33: An elastomer will fail to regain its
Q52: The average rate of disappearance of A
Q61: 0.7515 moles of nitrogen gas and 0.1135
Q87: Which statement about atmospheric pressure is false?<br>A)As
Q95: A solution containing 10.0 g of an
Q142: Which of the following liquids will have
Q153: The solubility of Ar in water at
Q164: The density of ammonia gas in a
Q176: The electron-domain geometry of _ is tetrahedral.<br>A)CBr<sub>4</sub><br>B)PH<sub>3</sub><br>C)CCl<sub>2</sub>Br<sub>2</sub><br>D)XeF<sub>4</sub><br>E)all