Calculate the freezing point of a 0.05500 m aqueous solution of NaN 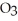 .The molal freezing-point-depression constant of water is
.The molal freezing-point-depression constant of water is 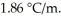 (Assume 100% ionization of NaNO3.)
(Assume 100% ionization of NaNO3.)
Definitions:
Prenatal Nutrition
The dietary and nutritional considerations and requirements during pregnancy, critical for the health and development of the fetus.
Embryo
An embryo is an early stage of development in multicellular organisms, in humans, it refers to the phase of prenatal development between conception and the fetal stage.
Cocaine
A powerful, addictive stimulant drug derived from the leaves of the coca plant, which is native to South America.
Stillbirth
The delivery of a baby who has died in the womb after 20 weeks of pregnancy, distinguished from earlier pregnancy loss, which is typically referred to as a miscarriage.
Q2: A face-centered cubic unit cell contains contains
Q4: A closed-end manometer was attached to a
Q39: Human blood is considered to be _.<br>A)neutral<br>B)very
Q64: What volume (mL)of fluorine gas is required
Q92: Dinitrogen tetroxide partially decomposes according to the
Q93: Which of the following salts will produce
Q95: A 0.22 M aqueous solution of the
Q113: A 30.0 g sample of potassium nitrate
Q124: A solution contains 30 ppm of some
Q134: Of the following,a 0.2 M aqueous solution