What is the freezing point (°C) of a solution prepared by dissolving 11.3 g of Ca(NO3) 2 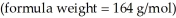 in 115 g of water? The molal freezing point depression constant for water is
in 115 g of water? The molal freezing point depression constant for water is 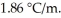 (Assume 100% ionization of Ca(NO3) 2.)
(Assume 100% ionization of Ca(NO3) 2.)
Definitions:
Involuntary Active Euthanasia
The intentional administration of lethal drugs or other means of producing a painless death without the person’s informed consent.
Informed Consent
The process through which individuals are provided with comprehensive details about a medical treatment or experiment before agreeing to participate.
Lethal Drugs
are substances capable of causing death when administered in a sufficient quantity or concentration.
Personal Fable
A cognitive distortion experienced during adolescence in which individuals believe themselves to be unique, invincible, and misunderstood.
Q6: Using the data in the table,which of
Q16: The equilibrium-constant expression depends on the _
Q19: Which group of transition metals have the
Q25: All of the following are classified as
Q26: The molarity of urea (MW = 60.0
Q51: The density of HCN is _ g/L
Q77: Which of the following is a condensation
Q93: Which of the following salts will produce
Q94: Of the following substances,an aqueous solution of
Q127: The effusion rate of a gas is