On a clear day at sea level,with a temperature of 25 °C,the partial pressure of 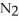 in air is 0.78 atm and the concentration of nitrogen in water is
in air is 0.78 atm and the concentration of nitrogen in water is 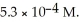 When the partial pressure of
When the partial pressure of 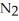 is ________ atm,the concentration in water is 2.0
is ________ atm,the concentration in water is 2.0 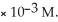
Definitions:
Q8: The base-dissociation constant,K<sub>b</sub>,for an unknown base is
Q9: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt=" decomposes
Q16: Which category of plastics will generally be
Q52: The average rate of disappearance of A
Q70: A water sample tested positive for lead
Q92: An aqueous solution with a concentration of
Q99: What is the mole fraction of LiCl
Q114: The pH of a 0.15 M aqueous
Q116: The decomposition of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The decomposition
Q133: 13.3 g of benzene (C<sub>6</sub>H<sub>6</sub>)is dissolved in