The half-life of a first-order reaction is 13 min.If the initial concentration of reactant is 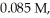 it takes
it takes  for it to decrease to 0.055 M.
for it to decrease to 0.055 M.
Definitions:
Negotiable
Capable of being transferred or exchanged through endorsement or delivery, usually in the context of financial instruments.
Occurrence
An event or incident that takes place, often used in legal and insurance contexts to specify an event covered by a policy or agreement.
Nonoccurrence
The failure or absence of an event or condition that was anticipated or required.
Event
A specific occurrence or happening, often of significance, that takes place at a particular time.
Q2: A face-centered cubic unit cell contains contains
Q11: Chemical treatment of municipal water supplies commonly
Q24: Calculate the freezing point of a solution
Q25: The dissociation energy of N<sub>2</sub> is 941
Q31: The value of K<sub>eq</sub> for the equilibrium
Q81: According to the phase diagram shown above,what
Q84: A solid has a very high melting
Q89: What is the rms speed (m/s)of oxygen
Q93: The majority of ozone that protects against
Q139: How many liters of O<sub>2</sub> are consumed