The half-life of a first-order reaction is 13 min.If the initial concentration of reactant is 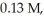 it takes
it takes  for it to decrease to 0.085 M.
for it to decrease to 0.085 M.
Definitions:
Interest
The cost of borrowing money or the return on investment capital, often expressed as a percentage rate over a period of time.
Adjusted Cash Balance
The cash balance recorded in accounting books after considering outstanding checks and deposits in transit, ensuring it matches the actual bank balance.
Check Register
A record book or electronic log used to track checks written, deposits made, and current account balances.
Bank Statement
A document or electronic record issued by a bank summarizing a customer's transactions and current balance over a specific period.
Q10: The enzyme _ converts nitrogen into ammonia.<br>A)oxygenase<br>B)hydrogenase<br>C)oxidase<br>D)nitroglycerine<br>E)nitrogenase
Q17: For the elementary reaction NO<sub>3</sub> + CO
Q21: A 25.0-mL sample of 0.150 M hydrocyanic
Q23: The critical temperature and pressure of CS<sub>2</sub>
Q23: What is the percent ionization of nitrous
Q26: In a solution,when the concentrations of a
Q38: The equilibrium constant for reaction 1 is
Q46: What is the mole fraction of hydrochloric
Q67: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of
Q98: Carbon dioxide contributes to atmospheric warming by