The half-life of a first-order reaction is 13 min.If the initial concentration of reactant is 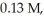 it takes
it takes  for it to decrease to 0.085 M.
for it to decrease to 0.085 M.
Definitions:
Nocturia
The need to wake up during the night in order to urinate, disrupting sleep.
Polyuria
a condition characterized by the production of abnormally large volumes of dilute urine, often a symptom of various medical conditions.
Oliguria
The medical term for producing abnormally small amounts of urine.
Dysuria
Painful or difficult urination, often indicating a urinary tract infection.
Q6: Which component of air is the primary
Q10: The enzyme _ converts nitrogen into ammonia.<br>A)oxygenase<br>B)hydrogenase<br>C)oxidase<br>D)nitroglycerine<br>E)nitrogenase
Q43: Define Beer's Law.
Q59: A second-order reaction has a half-life of
Q68: The dividing line between the troposphere and
Q91: For the endothermic reaction CaCO<sub>3</sub> (s) <img
Q108: If the partial pressure of oxygen in
Q109: - <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="- is
Q129: Under constant conditions,the half-life of a first-order
Q150: The most likely van't Hoff factor for