A sealed 1.0 L flask is charged with 0.500 mol of I2 and 0.500 mol of Br2.An equilibrium reaction ensues: I2 (g) + Br2 (g)  2IBr (g)
2IBr (g)
When the container contents achieve equilibrium,the flask contains 0.84 mol of IBr.The value of 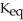 is ________.
is ________.
Definitions:
Portfolio Beta
A measure of the volatility of a portfolio compared to the market as a whole, indicating the expected percentage change in a portfolio's value for a 1% change in the market.
Alpha
A measure of the active return on an investment, gauging how much a portfolio or fund outperforms or underperforms its benchmark index.
Hedge Fund
An investment fund that employs various strategies to produce high returns, including leverage, short-selling, and derivatives, accessible typically to wealthy investors.
Incentive Bonus
A form of additional compensation paid to an employee as a reward for achieving certain goals or exceptional performance.
Q3: In which of the following aqueous solutions
Q17: What is the percent gold in a
Q23: Which one of the following is produced
Q41: What is the concentration (M)of HCl in
Q48: A result of the common-ion effect is
Q61: The value of ΔS° for the decomposition
Q69: The salinity,density,and temperature of seawater varies with
Q73: What is the primary buffer system that
Q94: What is the wavelength of a photon
Q95: A solution containing 10.0 g of an