Phosphorous Trichloride and Phosphorous Pentachloride Equilibrate in the Presence of Molecular
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: 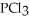 (g) +
(g) + 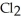 (g) →
(g) → 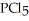 (g) An equilibrium mixture at 450 K contains
(g) An equilibrium mixture at 450 K contains 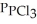 = 0.224 atm,
= 0.224 atm, 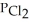 = 0.284 atm,and
= 0.284 atm,and 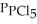 = 4.24 atm.What is the value of Kp at this temperature?
= 4.24 atm.What is the value of Kp at this temperature?
Definitions:
Active Bond Portfolio
A portfolio of bonds managed with the intention of outperforming the market through various strategies, such as sector rotation and duration management.
Financial Institutions
Entities that provide financial services, including banks, insurance companies, pension funds, brokerage firms, and others.
Realized Rate
The actual return earned on an investment over a given time period.
Investment Horizon
Investment horizon refers to the total length of time an investor expects to hold an investment or portfolio before liquidating it.
Q7: An unknown metal crystallizes in a body-centered
Q9: If the reaction quotient Q for a
Q20: The average rate of disappearance of A
Q25: Which of the following statements describes why
Q48: The mole fraction of nitrogen in dry
Q64: To produce acceptable quality drinking water from
Q69: In general,as activation energy increases,reaction rate _.<br>A)goes
Q101: What is the order of the reaction
Q103: On a clear day at sea level,with
Q108: Which of the following is arranged correctly