At elevated temperatures,molecular hydrogen and molecular bromine react to partially form hydrogen bromide: H2 (g) + Br2 (g)  2HBr (g)
2HBr (g)
A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of 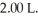 At equilibrium at 700 K,there are 0.546 mol of H2 present.At equilibrium,there are ________ mol of Br2 present in the reaction vessel.
At equilibrium at 700 K,there are 0.546 mol of H2 present.At equilibrium,there are ________ mol of Br2 present in the reaction vessel.
Definitions:
Brachialgia
Pain in the arm, specifically referring to nerve pain that can be caused by various conditions affecting the brachial plexus or surrounding nerves.
Arm
A limb on the human body extending from the shoulder to the wrist, including the forearm and upper arm.
Pain
A distressing feeling and emotional response linked to real or possible harm to the body, or defined by reference to such harm.
Anatomic Position
A standardized method of observing or imaging the body that allows precise and consistent anatomical references; typically, the person stands erect with the feet flat and arms at the sides with palms facing forward.
Q5: Define activation energy.
Q6: If a rate law is first order
Q18: The principal source of the difference in
Q30: Ethanol melts at -114 °C and boils
Q61: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of
Q65: Quantum dots can be found as particles
Q67: The equilibrium constant for the gas phase
Q100: A 0.14 M aqueous solution of the
Q105: An unsaturated solution is one that _.<br>A)has
Q110: What is the pH of a buffer