What is the pH of a buffer solution that is 0.172 M in hypochlorous acid (HClO) and 0.131 M in sodium hypochlorite? The 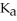 of hypochlorous acid is 3.8 ×
of hypochlorous acid is 3.8 × 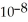 .
.
Definitions:
Apparent Authority
The appearance or assumption of authority based on the actions or statements of the principal, leading third parties to believe an agent has authority to act.
Actual Authority
A legal concept where a party is officially granted the power to act on behalf of another, particularly in a business setting.
Implied Authority
The power of an agent to act on behalf of a principal, assumed to be granted due to the nature of the relationship, without explicit consent.
Actual Authority
The specific powers that a principal explicitly grants to an agent to act on their behalf.
Q2: The major product of a _ fuel
Q12: The value of ΔG° at 181.0 °C
Q21: The rate constant for a second-order reaction
Q30: What is the conjugate acid of NH<sub>2</sub><sup>-</sup>?<br>A)NH<sub>2</sub><sup>+</sup><br>B)NH<sub>3</sub><sup>+</sup><br>C)NH<sub>4</sub><sup>+</sup><br>D)NH<sub>3</sub><br>E)NH<sub>4</sub>OH
Q83: 291.5 grams of calcium chloride is dissolved
Q88: Which element is oxidized in the reaction
Q97: The K<sub>a</sub> of acetic acid (HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)is <img
Q103: The second law of thermodynamics states that
Q116: Which of the following could be added
Q155: The solubility of oxygen gas in water