Consider the following reaction at equilibrium: 2N 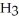 (g)
(g) 
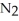 (g) + 3
(g) + 3 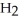 (g) ΔH° = +92.4 kJ
(g) ΔH° = +92.4 kJ
Le Châtelier's principle predicts that removing 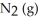 to the system at equilibrium will result in ________.
to the system at equilibrium will result in ________.
Definitions:
Instrumentality
In organizational psychology, the perceived relationship between performance and the attainment of certain outcomes.
Performance Accomplishment
Achieving specific goals or standards of work that are recognized as an indicator of competence.
Commutative Justice
focuses on fairness in the exchange between individuals, ensuring a balance of give and take.
Sexual Harassment Complaint
A formal report made by an individual alleging unwelcome sexual advances, requests for sexual favors, or other verbal or physical conduct of a sexual nature in the workplace or learning environment.
Q25: Of the following,_ is the most volatile.<br>A)C<sub>2</sub>H<sub>6</sub><br>B)C<sub>2</sub>I<sub>6</sub><br>C)C<sub>2</sub>Br<sub>6</sub><br>D)C<sub>2</sub>Cl<sub>6</sub><br>E)C<sub>2</sub>F<sub>6</sub>
Q34: Ethanol ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="Ethanol (
Q41: The conjugate base of CH<sub>3</sub>NH<sub>3</sub><sup>+</sup> is _.<br>A)CH<sub>3</sub>NH<sub>2</sub><sup>+</sup><br>B)CH<sub>3</sub>NH<sub>2</sub><sup>-</sup><br>C)CH<sub>3</sub>NH<sup>+</sup><br>D)CH<sub>3</sub>NH<sub>2</sub><br>E)none
Q42: What is the mass % of ammonium
Q49: Given the thermodynamic data in the table
Q80: List two of the three major sources
Q86: Which reaction will shift to the left
Q120: Calculate the enthalpy change (in kJ)associated with
Q126: Nitrogen dioxide decomposes to nitric oxide and
Q135: What is the formula weight of iron(III)chloride