The acid-dissociation constants of phosphoric acid (H3PO4) are 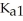 = 7.5 × 10-3,
= 7.5 × 10-3, 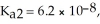 and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
Definitions:
Interests
Activities, subjects, or fields that attract attention and engagement from individuals or groups.
Confrontation and Problem Solving
Addressing and engaging in direct discussions or actions to resolve disputes, challenges, or issues.
Direct Reports
Employees who report directly to a manager or supervisor.
Conflict
Refers to a situation where there is a clash of interests, values, actions, views or directions between individuals or groups.
Q4: The average pH of the oceans is
Q12: What is the pH of an aqueous
Q22: Which of the following is true?<br>A)If we
Q23: The value of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The value
Q34: What is the molality of a 24.4%
Q44: A solution is prepared by dissolving 0.23
Q58: The value of ΔG° at 25 °C
Q84: A unimolecular elementary reaction involves _ reactant
Q115: The concentration of iodide ions in a
Q132: What is the conjugate acid of HCO<sub>3</sub><sup>-</sup>?<br>A)CO<sub>2</sub><sup>2-</sup><br>B)H<sub>2</sub>CO<sub>3</sub><br>C)HCO<sub>2</sub><sup>2-</sup><br>D)CO<sub>3</sub><sup>2-</sup><br>E)none