A solution is prepared by dissolving 0.23 mol of benzoic acid and 0.27 mol of sodium benzoate in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly.The pH does not increase drastically because the NaOH reacts with the ________ present in the buffer solution.The 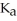 of benzoic acid is 6.3 × 10-5.
of benzoic acid is 6.3 × 10-5.
Definitions:
Insurance Companies
Organizations that provide insurance policies to consumers, covering a variety of risks in exchange for premiums.
Insurance Company
A business entity that provides financial protection against losses and risks in exchange for premiums.
Property Loss
The term refers to the loss or damage of property due to various risks such as fire, theft, or natural disasters.
Adverse Selection
A situation where asymmetric information leads to the selection of undesirable risks by one party in a contract.
Q15: The value of ΔH° for the oxidation
Q29: AgBr would have the lowest solubility in
Q29: What is the rate constant (s<sup>-1</sup>)for this
Q39: Pure solids and pure _ are excluded
Q42: The value of ΔG° at 25 °C
Q59: Show how a molecule of C<sub>2</sub>F<sub>2</sub>Cl<sub>2</sub> can
Q78: Which of the following has the largest
Q93: The half-life of <sup>222</sup>Rn is 3.80 days.If
Q97: The brown color of photochemical smog over
Q115: What is the mole fraction of N