The acid-dissociation constants of phosphoric acid (H3PO4) are 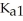 = 7.5 × 10-3,
= 7.5 × 10-3, 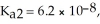 and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
Definitions:
Informal Social Interaction
Casual exchanges or engaging in activities with others without formal structure or organizational context.
Student Behavior
Refers to the range of actions and mannerisms exhibited by students in the context of learning environments, influencing the teaching and learning process.
Head Start
A program in the United States offering extensive early childhood education, health care, nutrition, and services that engage parents, targeting low-income families and their children.
Family Income
The total amount of money earned by members of a family, used as an indicator of the economic status of the family.
Q15: Calculate the mole fraction of phosphoric acid
Q28: The concentration of ozone in a sample
Q61: As one gains altitude in the atmosphere,based
Q69: The reduction half reaction occurring in the
Q69: Consider the following reaction at equilibrium. 2CO<sub>2</sub>
Q69: What is the molarity of phosphoric acid
Q70: The acid-dissociation constants of sulfurous acid (H<sub>2</sub>SO<sub>3</sub>)are
Q73: The equilibrium constant for the following reaction
Q83: How many moles of B are present
Q107: The rate of disappearance of HBr in