A solution is prepared by dissolving 0.23 mol of hypochlorous acid and 0.27 mol of sodium hypochlorite in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly.The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution.The 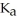 of hypochlorous acid is 1.36 × 10-3.
of hypochlorous acid is 1.36 × 10-3.
Definitions:
Early Adulthood
A stage of human development that occurs roughly between the ages of 18 to 25, marked by increased independence and responsibility.
Binge Drinking
Heavy episodic drinking; consuming five or more alcoholic beverages in one sitting for men and four drinks in one sitting for women.
Heavy Drinking
The consumption of large quantities of alcohol in a short period, often leading to health risks and addiction.
College Students
Individuals enrolled in institutions of higher education to pursue post-secondary degrees or certificates.
Q15: What is the molar solubility of silver
Q19: If a reaction is endothermic,_ the reaction
Q27: According to the Arrhenius concept,an acid is
Q45: The magnitude of the rate constant is
Q46: Based on standard reduction potentials,the most difficult
Q60: This reaction is an example of _.
Q103: On a clear day at sea level,with
Q110: The missing reactant from this reaction is
Q115: Clean rainwater is _ mainly due to
Q125: The "scale" caused by hard water is