Calculate the pH of a solution prepared by dissolving 0.270 mol of weak acid HA and 0.260 mol of its conjugate base in water sufficient to yield 1.00 L of solution.The 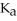 of HA is
of HA is 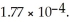
Definitions:
Labor Unions
Organizations formed by workers in various sectors to protect and advance their rights, wages, benefits, and working conditions through collective bargaining.
Generation X
The demographic cohort following the Baby Boomers, typically defined as individuals born between the early-to-mid 1960s and the late 1970s to early 1980s.
Baby Boomers
Refers to the generation born approximately between 1946 and 1964, experiencing significant population growth following World War II.
Wagner Act
A foundational piece of U.S. labor law enacted in 1935 that protects workers' rights to form unions and engage in collective bargaining.
Q7: The expression of K<sub>eq</sub> for the following
Q16: The concentration of carbon monoxide in a
Q29: What is the coefficient of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q32: How many milliliters of 0.120 M NaOH
Q36: The pH of a solution prepared by
Q64: Dinitrogentetraoxide partially decomposes into nitrogen dioxide.A 1.00-L
Q92: Dinitrogen tetroxide partially decomposes according to the
Q121: Given the following table of thermodynamic data,
Q132: What is the molal concentration of KCl
Q139: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The for