A 25.0-mL sample of 0.150 M hypochlorous acid is titrated with a 0.150 M NaOH solution.What is the pH after 13.3 mL of base is added? The 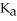 of hypochlorous acid is 3.0 × 10-8.
of hypochlorous acid is 3.0 × 10-8.
Definitions:
Hours Worked
The total number of hours spent working by an individual or group in a specific period.
Statistics Students
Individuals engaged in the study of statistics, which includes the collection, analysis, interpretation, and presentation of data.
Frequency Distribution
A summary of data that shows the number of occurrences of each value or category of a variable.
Cumulative Frequency
The sum of a given frequency and all frequencies so far in a frequency distribution.
Q14: A solution containing which one of the
Q61: The freezing point of ethanol ( <img
Q64: Transuranium elements have atomic numbers greater than
Q78: Which of the following has the largest
Q83: 291.5 grams of calcium chloride is dissolved
Q86: The decomposition of [A] in solution at
Q87: The balanced half-reaction in which dichromate ion
Q126: Which of the following acids will be
Q132: The main scientific difficulty in achieving a
Q135: The half-life for beta decay of strontium-90