Calculate the pH of a solution that is 0.322 M in nitrous acid ( 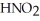 ) and 0.178 M in potassium nitrite (KNO2) .The acid dissociation constant of nitrous acid is 4.50 ×
) and 0.178 M in potassium nitrite (KNO2) .The acid dissociation constant of nitrous acid is 4.50 × 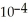 .
.
Definitions:
Photoreceptor
Specialized retinal neuron that transduces light into neural activity.
Circadian Rhythms
Natural, internal processes that regulate the sleep-wake cycle and repeat roughly every 24 hours, influencing physical, mental, and behavioral changes.
Retinohypothalamic Tract
A neural pathway that carries information from the retina to the hypothalamus, involved in regulating circadian rhythms.
Circadian Rhythms
The natural, internal processes that regulate the sleep-wake cycle and repeat roughly every 24 hours, influencing physical, mental, and behavioral changes.
Q13: In terms of binding energy per nucleon,what
Q24: Which of the following statements is false?<br>A)The
Q33: At 900.0 K,the equilibrium constant (K<sub>p</sub>)for the
Q39: Pure solids and pure _ are excluded
Q52: The equilibrium expression for K<sub>p</sub> for the
Q58: The half-reaction occurring at the cathode in
Q87: The balanced half-reaction in which dichromate ion
Q119: What radioactive element is used to diagnose
Q130: What is the atomic number of a
Q161: All atoms of a given element have