The value of ΔG° at 181.0 °C for the formation of calcium chloride from calcium metal and chlorine gas is ________ kJ/mol.At 25.0 °C for this reaction,ΔH° is -795.8 kJ/mol,ΔG° is -748.1 kJ/mol,and 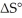 is
is 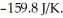
Definitions:
Paper Bag
A simple container made of paper, typically used for carrying items or packaging.
Reflex Hammer
A medical tool used by healthcare professionals to test deep tendon reflexes to check for abnormalities of the nervous system.
Neurological System
The part of an organism that coordinates its actions by transmitting signals to and from different parts of its body, encompassing the brain and spinal cord.
Tendons
Tough, fibrous connective tissues that connect muscles to bones, enabling movement.
Q14: Which of the halogens in Table 20.1
Q24: Consider the following reaction at equilibrium: 2C
Q26: The standard cell potential (E°<sub>cell</sub>)of the reaction
Q55: The mode of decay of <sup>32</sup>P is
Q56: A substance that is capable of acting
Q69: How many milliliters of 0.0839 M NaOH
Q87: Classify the following compounds as weak acids
Q87: The balanced half-reaction in which dichromate ion
Q103: The oxidation state of oxygen in O<sub>2</sub>F<sub>2</sub>
Q114: The value of ΔS° for the catalytic