A buffer solution with a pH of 4.31 is prepared with 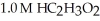 and
and 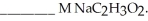 The Ka of H
The Ka of H 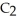
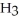
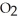 is
is 
Definitions:
Brand Image
The public's perception and emotional appeal of a brand as a result of its branding efforts, which may include product experience, advertising, design, and media portrayal.
Endorsement Deal
A contractual agreement where a brand pays an individual to use, promote, and recommend its products or services, often leveraging the individual's fame or expertise.
Jeremy Lin
A professional basketball player known for his success and significant impact in the NBA, particularly during the "Linsanity" period with the New York Knicks.
Specific Attributes
Particular characteristics or qualities that distinguish objects, individuals, or concepts from others within a group or category.
Q2: The solubility product of a compound is
Q6: The standard cell potential (E°<sub>cell</sub>)of the reaction
Q43: The pH of a solution prepared by
Q47: Of the compounds below,a 0.1 M aqueous
Q57: <sup>41</sup>Ca decays by electron capture.The product of
Q77: Which one of the following pairs cannot
Q80: List two of the three major sources
Q99: What is the change in entropy in
Q111: Sulfur compounds in the atmosphere are equally
Q122: The combustion of acetylene in the presence