The value of ΔG° at 261.0 °C for the formation of phosphorous trichloride from its constituent elements, 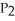 (g) +
(g) + 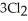 (g) →
(g) → 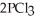 (g) is ________ kJ/mol.At 25.0 °C for this reaction,ΔH° is -720.5 kJ/mol,ΔG° is
(g) is ________ kJ/mol.At 25.0 °C for this reaction,ΔH° is -720.5 kJ/mol,ΔG° is 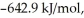 and
and 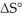 is
is 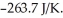
Definitions:
Future Spot Rate
The anticipated exchange rate at which one currency will be exchanged for another in the spot market at a future date.
Cash Flow Hedge
A risk management technique used by companies to protect against the risk associated with fluctuations in cash flows, typically related to future interest or currency exchange rates.
Canadian Dollars
The official currency of Canada, often represented by the symbol CAD or sometimes C$ to distinguish it from other dollar-denominated currencies.
Foreign Currency Put Option
A financial derivative that gives the holder the right, but not the obligation, to sell a specific amount of foreign currency at a set price within a specified time period.
Q18: The Henderson-Hasselbalch equation is _.<br>A)[H<sup>+</sup>] = K<sub>a</sub>
Q24: Consider the following reaction at equilibrium: 2C
Q25: Consider the reaction between ammonia and hydrochloric
Q36: The standard emf for the cell using
Q37: Which one of the following is always
Q41: Municipal water treatment consists of five steps
Q96: Consider the reaction: FeO (s)+ Fe (s)+
Q111: What is the pH of a solution
Q125: The value of ΔS° for the decomposition
Q132: What is the conjugate acid of HCO<sub>3</sub><sup>-</sup>?<br>A)CO<sub>2</sub><sup>2-</sup><br>B)H<sub>2</sub>CO<sub>3</sub><br>C)HCO<sub>2</sub><sup>2-</sup><br>D)CO<sub>3</sub><sup>2-</sup><br>E)none