For a given reaction,ΔH = +74.6 kJ/mol,and the reaction is spontaneous at temperatures above the crossover temperature, 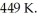 The value of
The value of  assuming that ΔH and ΔS do not vary with temperature.
assuming that ΔH and ΔS do not vary with temperature.
Definitions:
China
A country in East Asia, the world's most populous nation, with a rich history, diverse cultures, and significant influence on global affairs.
Japan
Situated in the Pacific, Japan stands as a nation steeped in a blend of modern innovation alongside deep-rooted cultural practices and is noted for its significant contributions to global culture and technology.
Emelian Pugachev
A Cossack leader who led a major peasant uprising against the Russian empire in the 18th century, known as Pugachev's Rebellion.
Tsar Peter III
The Russian emperor who reigned briefly in 1762 and was noted for his attempts to modernize the Russian army and government before being overthrown.
Q11: Chemical treatment of municipal water supplies commonly
Q49: Calculate the pH of a solution that
Q65: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt=" acid and
Q99: Calculate the molarity of hydroxide ion in
Q106: The transmutation in which a curium-242 nucleus
Q108: Which of the following equations correctly represents
Q112: What is the pOH of an aqueous
Q121: What is the missing product from this
Q122: Which of the following is an ionic
Q128: The missing product from this reaction is