For a given reaction,ΔS = +69.0 J/mol∙K,and the reaction is spontaneous at temperatures above the crossover temperature, 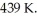 The value of
The value of  assuming that ΔH and ΔS do not vary with temperature.
assuming that ΔH and ΔS do not vary with temperature.
Definitions:
Art of Persuasion
The skill of influencing or convincing others to adopt a particular view or take a specific action.
Rancor
Bitterness or resentfulness, especially when long-standing; it can significantly impact personal and professional relationships.
High Performance
Exceptionally strong output or results from a person, group, system, or organization, often exceeding the standard expectations.
Leadership Principles
Fundamental guidelines that inform and shape the actions and behaviors of leaders in various contexts.
Q31: A solution is prepared by dissolving 0.23
Q54: In the gas phase reaction below,NH<sub>3</sub> is
Q61: A borane is a _.<br>A)compound containing only
Q69: The salinity,density,and temperature of seawater varies with
Q95: Cesium-131 has a half-life of 9.7 days.What
Q98: Which of the following reactions would have
Q111: The entropy of the universe is _.<br>A)constant<br>B)continually
Q118: Which pair of formula/name is incorrect?<br>A)NO<sub>2</sub> /
Q128: What is the pOH of an aqueous
Q142: Which equation correctly represents the reaction between