The standard cell potential (E°cell) of the reaction below is -0.34 V.The value of 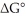 for the reaction is ________ kJ/mol. Cu (s) + 2H+ (aq) → Cu2+ (aq) + H2 (g)
for the reaction is ________ kJ/mol. Cu (s) + 2H+ (aq) → Cu2+ (aq) + H2 (g)
Definitions:
Gender Role Stereotypes
Preconceived ideas about the behaviors, attitudes, and characteristics that society considers appropriate for men and women.
Higher Goals
Objectives or ambitions that are more significant and challenging, aimed at achieving a higher purpose or greater success.
Daughters
Female offspring in relation to their parents.
Transgendered People
Individuals whose gender identity or expression differs from the sex they were assigned at birth; may include those who have transitioned to the gender they identify with or wish to do so.
Q12: A voltaic cell is constructed with two
Q21: _ are typically basic while _ are
Q29: The belt of nuclear stability ends with
Q44: A solution is prepared by dissolving 0.23
Q50: Define the Nernst equation.
Q52: Of the following,which is the strongest acid?<br>A)HIO<sub>4</sub><br>B)HIO<sub>3</sub><br>C)HIO<sub>2</sub><br>D)HIO<br>E)The
Q56: Which noble gas is known to form
Q69: How many milliliters of 0.0839 M NaOH
Q101: The balanced half-reaction in which sulfate ion
Q114: The value of ΔS° for the catalytic