Adding more solid silver chloride to a saturated aqueous solution of silver chloride will shift the equilibrium to the right. 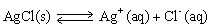
Definitions:
Note
A brief record used to aid the memory or for future reference.
Mechanism
The underlying process or system that enables something to function or produces a particular outcome.
Mediation
The process through which tools organize people’s activities and ways of relating to their environments.
Q10: Calculate the percent ionization of a 0.200
Q15: Air is a substance.
Q16: At room temperature fats exist as<br>A)solids.<br>B)liquids.<br>C)gases.<br>D)vapors.
Q25: In order to decrease the freezing point
Q30: What type of bond exists within the
Q39: What is the mass percent of a
Q51: What type of emission causes Ra-226 to
Q71: In which direction will the point of
Q80: As the pressure of a sample of
Q84: The general formula for a ketone is<br>A)RCHO<br>B)ROR<br>C)RCOOR<br>D)R<sub>2</sub>CO