Multiple Choice
The boiling point of 1,4-butanediol is 230°C. Would you expect this compound to be soluble or insoluble in room-temperature water? 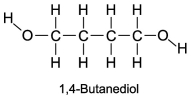
Definitions:
Related Questions
Q11: What is the molecular shape of the
Q49: What happens to the entropy of a
Q52: An inventor claims to have developed a
Q74: Which type of radiation is being emitted
Q79: Why does water not freeze at 0°C
Q88: If it takes three golf balls to
Q91: How would you classify the following material?
Q109: What property of alloys make them ideal
Q114: An element found in another galaxy exists
Q123: The nucleus of an electrically neutral iron