Why is calcium chloride, 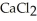 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?
Definitions:
Deviant
Behavior that violates the norms, values, or expectations of a group or society, which can provoke negative or positive reactions from others.
Murder
The unlawful premeditated killing of one human being by another.
Crimes
Acts that violate legal statutes and are punishable by the state or other authority.
Gains
Increases or improvements in various contexts, such as health, wealth, knowledge, or efficiency.
Q8: Water is formed from the reaction of
Q18: If a neutral atom gains two electrons,
Q34: Which of the above would best describe
Q70: In a battery, the following oxidation-reduction reactions
Q79: Why does water not freeze at 0°C
Q93: The number of nonbonding pairs in the
Q112: Which of the following statements about radiation
Q128: Which of the beams is actually composed
Q130: What is the main characteristic of a
Q143: The shell model presented in this book