Why is the formation of iron hydroxide, Fe 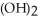 , from
, from 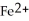 and O
and O 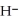 not considered an oxidation-reduction reaction?
not considered an oxidation-reduction reaction?
Definitions:
Reasonable Advance Notice
Notice given well in advance of an action, allowing the recipient enough time to prepare or respond appropriately.
Indefinite Period
A time frame without a specified end date, often used in contracts or employment agreements.
Security Deposit
A sum of money held in trust as protection against potential damage or default, typically paid by a tenant to a landlord.
Ordinary Wear
The natural and expected deterioration of an item over time due to normal and reasonable use.
Q6: The following set of redox reactions takes
Q23: What is the main difference between the
Q25: Water has a high specific heat because
Q47: In the following buffer system, what happens
Q49: Why do water drops bead on a
Q54: Describe which level of the food chain
Q63: Would fevers run higher or lower if
Q68: Common drugs originate from _.<br>A) natural products<br>B)
Q109: How many structural isomers are there for
Q115: How readily an acid donates a hydrogen