What element is oxidized in the following equation and what element is reduced? 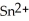 + 2 Ag → Sn + 2 Ag⁺
+ 2 Ag → Sn + 2 Ag⁺
Definitions:
Same-Sex Attraction
A form of sexual orientation characterized by a romantic or sexual attraction to individuals of the same sex.
Sexual Diversity
The range of differences in sexual orientation, identity, and practices among individuals and cultures.
Individual Inferiority
Individual inferiority describes feelings of being less valuable, competent, or worthy compared to others, often affecting self-esteem and behavior.
Social Interest
A sense of belonging and contributing to the well-being of others and society.
Q13: If champagne has a proof of 28,
Q17: Dip a paper clip into water and
Q38: Which of the following might best describe
Q41: Which of the following molecules could be
Q44: What is a chemical reaction?<br>A) when one
Q50: Why do changes in pH interfere with
Q83: What is the temperature change if you
Q101: Fat molecules do not have _.<br>A) a
Q114: What is the main function of the
Q121: Why are children more susceptible to vitamin