A substance has a melting point of 20°C and a heat of fusion of 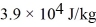 .The boiling point is
.The boiling point is 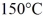 and the heat of vaporization is
and the heat of vaporization is 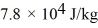 at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 600 J/(kg • K) ,1000 J/(kg • K) ,and 400 J/(kg • K) ,respectively.The quantity of heat required to raise the temperature of
at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 600 J/(kg • K) ,1000 J/(kg • K) ,and 400 J/(kg • K) ,respectively.The quantity of heat required to raise the temperature of 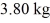 of the substance from
of the substance from 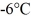 to
to 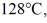 at a pressure of 1.0 atm,is closest to
at a pressure of 1.0 atm,is closest to
Definitions:
Medicaid
A public health insurance program in the United States providing health coverage to people with low incomes, including some low-income adults, children, pregnant women, elderly adults, and people with disabilities.
Federal program
A government initiative at the national level that provides services, funding, or support to the public in various areas such as education, health, and infrastructure.
Healthcare costs
The expenses associated with medical care, including the costs of hospitalization, medication, preventive treatments, and other health services.
Cognitive development
The process of growth and change in intellectual/mental abilities such as thinking, reasoning, and understanding across the lifespan.
Q7: A 1.25-kg ball begins rolling from rest
Q10: In the figure Q = 5.8 nC
Q10: A Carnot refrigerator has a coefficient of
Q22: In the figure,a mass of 31.77 kg
Q24: A 2.00-m long piano wire with a
Q26: The filament in a light bulb has
Q27: A 0.28-kg block on a horizontal frictionless
Q31: An atomic nucleus has a charge of
Q52: Consider a pipe of length L that
Q60: A policeman in a stationary car measures