A substance has a melting point of 20°C and a heat of fusion of 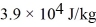 .The boiling point is
.The boiling point is 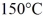 and the heat of vaporization is
and the heat of vaporization is 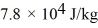 at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 600 J/(kg • K) ,1000 J/(kg • K) ,and 400 J/(kg • K) ,respectively.The quantity of heat required to raise the temperature of
at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 600 J/(kg • K) ,1000 J/(kg • K) ,and 400 J/(kg • K) ,respectively.The quantity of heat required to raise the temperature of 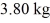 of the substance from
of the substance from 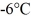 to
to 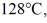 at a pressure of 1.0 atm,is closest to
at a pressure of 1.0 atm,is closest to
Definitions:
Analytical
Relating to the systematic examination of the structure of something, typically by separating it into its components to understand its nature or to find patterns.
Logical Problem Solving
The method of using structured reasoning to find solutions to specific issues or challenges.
Strengths-Based Leadership
A leadership approach focusing on identifying and maximizing the strengths of individuals and teams to achieve organizational goals.
Social Leadership
Group-oriented leadership that builds teamwork, mediates conflict, and offers support.
Q1: A substance has a melting point of
Q4: You are given a copper bar of
Q12: A small-sized 155-kg mass is located 1.50
Q28: The number of molecules in one mole
Q33: A very long nonconducting cylinder of diameter
Q35: The network shown in the figure is
Q39: Consider the motion of a 1.00-kg particle
Q42: A 25 kg object is undergoing lightly
Q44: Heat is added to a pure substance
Q49: As a tile falls from the roof