If you add 700 kJ of heat to 700 g of water at 70.0°C,how much water is left in the container? The latent heat of vaporization of water is 2.26 × 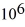 J/kg and its specific heat is is
J/kg and its specific heat is is 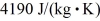 .
.
Definitions:
Systemic Impediments
Obstacles at the systemic level, such as policies or societal norms, that hinder progress or the attainment of equality.
Appropriate Tactics
Suitable strategies or methods employed to achieve a specific goal, especially within a professional or therapeutic context.
Facilitate Connections
The act of making it easier for individuals to establish relationships, networks, or links with others or necessary resources.
Community Helpers
Individuals who provide essential services and support within a community, such as firefighters, police officers, and healthcare workers.
Q4: In the figure,a block of mass m
Q6: A 120-kg refrigerator,2.00 m tall and 85.0
Q13: If the intensity level at distance d
Q14: How many grams of ice at -13°C
Q17: Suppose a region of space has a
Q18: Some properties of glass are listed here.
Q22: A 7.8-kg solid sphere,made of metal whose
Q26: One of the dangers of tornados and
Q49: If the electric field is zero everywhere
Q51: An extremely long thin wire carries a