Assuming the radius of diatomic molecules is approximately 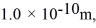 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 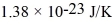 ,Avogadro's number is
,Avogadro's number is 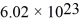 molecules/mole,and the ideal gas constant is
molecules/mole,and the ideal gas constant is 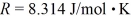 =
= 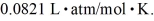
Definitions:
Production
The process of creating goods and services through the combination of labor, materials, and machinery.
Subcontracting
The practice of assigning or outsourcing part of the obligations and tasks of a contract to another party, often used to leverage specialized skills or cost advantages.
Inventory
The total amount of goods or materials held in stock by a business, available for sale or use in production.
Stockouts
Occurs when a product is not available in inventory for sale or use, leading to potential loss of sales and customer dissatisfaction.
Q5: The graph in the figure shows the
Q23: A machinist turns the power on to
Q26: A doubly charged ion (charge 2e)with velocity
Q29: Two identical ladders are 3.0 m long
Q33: As more resistors are added in parallel
Q34: A small sphere with a mass of
Q43: The entropy of an isolated system must
Q45: A 12,000-N car is raised using a
Q45: Three identical very small 50-kg masses are
Q49: If the electric field is zero everywhere