What is the average translational kinetic energy per molecule of an ideal gas at a temperature of 300 K? The Boltzmann constant is 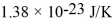 .
.
Definitions:
External Stimuli
Elements in the environment that can elicit responses from our sensory organs, influencing behavior or reactions.
Cognitive Perspective
A psychological approach that focuses on how we think, perceive, and solve problems, emphasizing mental processes.
Culture Emphasizes
The prioritization of certain values, beliefs, and practices by a society or group that shapes its members' behavior and perceptions.
Inconsistencies
Discrepancies or lack of alignment in information, actions, or beliefs that may lead to confusion or misunderstanding.
Q6: A 120-kg refrigerator,2.00 m tall and 85.0
Q8: The figure shows the displacement y of
Q9: A uniform solid sphere of mass M
Q13: Five capacitors are connected across a potential
Q13: The second law of thermodynamics leads us
Q20: A turntable has a radius of 0.80
Q25: A uniform solid sphere of mass 1.5
Q29: A metal bar is hanging from a
Q42: A satellite in a circular orbit of
Q60: A solid concrete wall 4.0 m by