A sealed container holds 0.020 moles of nitrogen (N2) gas,at a pressure of 1.5 atmospheres and a temperature of 290 K.The atomic mass of nitrogen is 14.0 g/mol.The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is 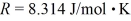 =
= 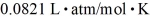 .The mass density of the gas is closest to
.The mass density of the gas is closest to
Definitions:
Denominator Level
In cost accounting, it’s the level of activity used to allocate fixed costs to products or services, often representing the production capacity.
Budget Variance
This is the difference between the budgeted or planned amount of expense or revenue, and the actual amount incurred or received.
Standard Hours
The set amount of time expected to complete a job or task, often used for planning and assessing the efficiency of operations.
Volume Variance
The difference between the budgeted volume of production and the actual volume, which affects the fixed overhead costs.
Q2: A charge of 2.00 μC flows onto
Q8: In the figure,when the terminal voltage V<sub>ab</sub>
Q11: A hollow conducting spherical shell has radii
Q12: A silver wire has a cross sectional
Q18: An ideal gas increases in temperature from
Q28: When a gas undergoes an isothermal process,there
Q28: The sound level at 1.0 m from
Q34: If we double only the mass of
Q38: A 2.00-kg object traveling east at 20.0
Q40: An aluminum wire and a steel wire,each