Sometimes an experiment requires a certain pure gas to be used at reduced pressure.One way to achieve this is to purchase a sealed glass container filled with the gas,and to introduce the gas into a vacuum by attaching the glass container to the vacuum chamber and breaking the tip of the glass container using a metallic bean and a magnet.If the volume of the glass container is 1.0 L and it is at a pressure of 1.0 × 105 Pa and if the vacuum chamber has a volume of 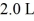 ,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
Definitions:
Stock B
Typically a classification indicating a type of stock with specific rights or characteristics, such as differing voting rights from Stock A in the same company.
Market Risk Premium
The extra profit an investor is aiming for by choosing to invest in a market portfolio that carries risk instead of opting for assets devoid of any risk.
Government Bond
A type of investment where an investor loans money to a government in exchange for periodic interest payments plus the return of the bond's face value at maturity.
Q1: A substance has a melting point of
Q6: Two motors in a factory are running
Q18: Some properties of glass are listed here.
Q23: A pipe that is 20.0 m long
Q26: Under electrostatic conditions,the electric field just outside
Q28: A tire is rolling along a road,without
Q29: What is the maximum current that can
Q35: The temperature of an ideal gas in
Q41: A very light 1.00-m wire consists of
Q49: The average molecular kinetic energy of a