How many moles of water (H2O) molecules are in a 4.00 m3 container at a pressure 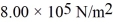 and temperature 600°C? The ideal gas constant is
and temperature 600°C? The ideal gas constant is 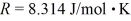 =
= 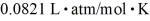 .
.
Definitions:
Accumulation
The process of gradually increasing or gathering together an amount of something over time.
Inhibition
The psychological process that restrains or controls an individual's thoughts, emotions, or actions.
Attention
The cognitive process of selectively concentrating on one aspect of the environment while ignoring other things.
Situational Demands
External pressures or circumstances that necessitate a response or adjustment from an individual or group.
Q2: If a certain sample of an ideal
Q9: A certain planet has an escape speed
Q16: A jet aircraft,in level flight at constant
Q37: A point charge Q = -500 nC
Q37: A half-ring (semicircle)of uniformly distributed charge Q
Q43: A 5.0-μC point charge is placed at
Q45: A negative charge,if free,will tend to move<br>A)
Q49: A tube of mercury with resistivity 9.84
Q52: Consider a pipe of length L that
Q58: A 5.0-liter gas tank holds 1.4 moles