What is the average translational kinetic energy per molecule of an ideal gas at a temperature of 300 K? The Boltzmann constant is 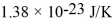 .
.
Definitions:
Influence
The capacity to have an effect on the character, development, or behavior of someone or something, or the effect itself.
Sigmund Freud
An Austrian neurologist who is the founding father of psychoanalysis, a method for treating psychopathology through dialogue between a patient and a psychoanalyst.
Unconscious
A part of the mind that houses thoughts, memories, and desires not within conscious awareness, a concept central to psychoanalytic theories.
Neurotic
Refers to an individual experiencing emotional distress but not losing touch with reality, often resulting from underlying stress or anxiety.
Q6: Two very large parallel sheets a distance
Q8: The figure shows the displacement y of
Q12: A silver wire has a cross sectional
Q13: If you double the pressure on the
Q16: Two experimental runs are performed to determine
Q21: The angular velocity of a 755-g wheel
Q27: The vertical displacement y(x,t)of a string stretched
Q42: Two loudspeakers placed 6.0 m apart are
Q47: Two very long parallel wires in the
Q53: A conducting sphere of radius 20.0 cm