What is the mean free path for the molecules in an ideal gas when the pressure is 100 kPa and the temperature is 300 K given that the collision cross-section for the molecules of that gas is 2.0 × 10-20 m2? The Boltzmann constant is 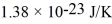 ,Avogadro's number is
,Avogadro's number is 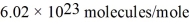 ,and the ideal gas constant is
,and the ideal gas constant is 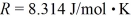 =
= 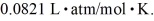
Definitions:
Direct Method
A method for creating a cash flow statement that itemizes the principal categories of gross cash inflows and outflows.
Cash Dividends
A portion of a company's earnings that is paid out to shareholders, typically in the form of cash.
Indirect Method
A approach used in cash flow statements to adjust net income for changes in non-cash accounts to determine net cash from operating activities.
Operating Activities
Operating activities include the primary revenue-generating activities of an organization as reflected in its cash flow.
Q7: A heavy boy and a lightweight girl
Q8: A 2.0 kg block on a frictionless
Q16: Water is flowing in a horizontal pipe
Q16: In a thermodynamic process involving 7.8 moles
Q39: In the figure,charge <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="In the
Q48: One very small uniformly charged plastic ball
Q50: A string 40.0 cm long of mass
Q55: A sample of an ideal gas is
Q55: A person makes ice tea by adding
Q61: A certain source of sound waves radiates