In a thermodynamic process involving 7.8 moles of an ideal gas,the gas is at an initial temperature of 24°C and has an initial volume of 0.040 m3.The gas expands adiabatically to a volume of 0.080 m3.For this gas,CV = 12.27 J/mol • K,and the ideal gas constant is 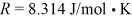 .Calculate the work done by the gas during this expansion.
.Calculate the work done by the gas during this expansion.
Definitions:
Queuing Systems
A mathematical concept used to describe and analyze the behavior of waiting lines or queues.
FCFS System
"First-Come, First-Served," a queue management strategy where requests are processed in the order they were received.
Arrival Rate
In queuing theory, it is the average number of items or people arriving at a service facility within a specified period of time.
Service Rate
Service rate is a measure used in service and operations management to denote the speed at which service providers can serve customers or process transactions.
Q8: A 2.00 kg piece of lead at
Q11: A uniform 300-kg beam,6.00 m long,is freely
Q15: A solid steel sphere with a radius
Q27: What is the minimum magnitude of an
Q47: In the circuit shown in the figure,all
Q50: An ideal gas in a balloon is
Q50: The x component of the velocity of
Q51: Two long parallel wires carry currents of
Q52: When a 20.0-ohm resistor is connected across
Q52: Consider a pipe of length L that