Assuming the radius of diatomic molecules is approximately 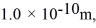 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 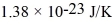 ,Avogadro's number is
,Avogadro's number is 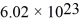 molecules/mole,and the ideal gas constant is
molecules/mole,and the ideal gas constant is 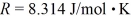 =
= 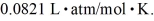
Definitions:
Total Variable Cost
Total variable cost is the sum of all costs that vary directly with the level of production or output, such as materials and labor.
Marginal Cost
The added cost of producing one additional unit of a product or service.
AVC
Average Variable Cost, calculated by dividing total variable cost by the quantity of output produced.
AP
If this refers to 'Average Product', it measures the output produced per unit of an input, averaging the total production over units of input. If it's another concept, additional context is needed for an accurate definition.
Q4: As shown in the figure,a container has
Q10: Two compressible solids are formed into spheres
Q12: What is the maximum length of a
Q13: In the figure,a small spherical insulator of
Q15: The graph in the figure shows the
Q17: A 2.00-kg object is attached to an
Q24: A 3.00-kg ball rests in a frictionless
Q27: A circular loop of wire of radius
Q33: A Carnot engine operating between a reservoir
Q52: The figure shows two long,parallel current-carrying wires.The