The temperature of an ideal gas in a sealed 0.40- 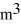 rigid container is reduced from 350 K to
rigid container is reduced from 350 K to 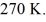 The final pressure of the gas is
The final pressure of the gas is 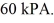 The molar heat capacity at constant volume of the gas is 28.0 J/mol • K.The heat absorbed by the gas is closest to
The molar heat capacity at constant volume of the gas is 28.0 J/mol • K.The heat absorbed by the gas is closest to
Definitions:
Equally Weighted Index
An equally weighted index is a stock market index in which each constituent stock contributes equally to the index's value, regardless of the company's market capitalization.
Value-Weighted Index
A stock market index in which each component is weighted in proportion to its market value.
Price-Weighted Index
A stock market index in which each company's influence on the index's movement is proportional to its stock price.
Bond Price Index
An index that measures the price changes of a specific set of bonds, reflecting overall movements in the bond market.
Q4: A Carnot engine operates between reservoirs at
Q4: By how many newtons does the weight
Q7: A 400-W computer (including the monitor)is turned
Q10: In the figure Q = 5.8 nC
Q11: A tiny object carrying a charge of
Q11: A 6.00-μF parallel-plate capacitor has charges of
Q18: An electron is initially moving to the
Q21: An ideal gas is kept in a
Q35: A +7.00 μC point charge and -9.00
Q36: Two harmonic sound waves reach an observer