An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram.The initial pressure is 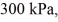 the initial volume is
the initial volume is 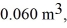 and the initial temperature is
and the initial temperature is 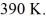 The ideal gas constant is
The ideal gas constant is 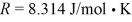 .The final pressure is
.The final pressure is 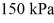 and the final temperature is
and the final temperature is 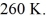 The work done by the gas is closest to
The work done by the gas is closest to
Definitions:
Profitability Ratios
Financial ratios that are used to measure the ability of a company to turn sales into profits and to earn profits on assets and owner’s equity committed.
Primary Financial Records
Essential documents that record and detail the financial activities and condition of a business or individual, such as income statements, balance sheets, and cash flow statements.
Benchmarking
The process of comparing one's business processes and performance metrics to industry bests or best practices from other industries.
Financial Ratios
Quantitative measures derived from financial statements used to evaluate a company's financial performance and condition.
Q2: A guitar string is fixed at both
Q5: Air is flowing through a rocket nozzle.Inside
Q8: The graph in the figure shows the
Q23: A turntable has a radius of 0.80
Q24: What is the mass density of argon
Q26: If the torque on an object adds
Q39: A circular loop of diameter 10 cm,carrying
Q40: A heat engine with an efficiency of
Q43: A sealed 89- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="A sealed
Q55: A sample of an ideal gas is