An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram.The initial pressure is 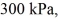 the initial volume is
the initial volume is 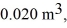 and the initial temperature is
and the initial temperature is 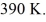 The final pressure is
The final pressure is 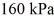 and the final temperature is
and the final temperature is 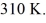 The change in the internal (thermal) energy of the gas is closest to
The change in the internal (thermal) energy of the gas is closest to
Definitions:
Rigorous
Characterized by a strict attention to detail; thorough and exacting.
Standard
A set of guidelines or principles established as a basis for comparison or measurement in quality assessment, production, and compliance.
Budget
A financial plan for a defined period, outlining an organization's projected revenues, expenses, and capital expenditures.
Standard Costs
Pre-determined or estimated costs of manufacturing, selling, or performing a service under normal conditions.
Q3: In the figure,a straight wire carries a
Q3: A frictionless pendulum released from 65 degrees
Q14: A large magnetic flux change through a
Q15: An ideal reversible refrigerator keeps its inside
Q16: You are generating traveling waves on a
Q26: The figure shows two tiny 5.0-g spheres
Q36: A solid metal sphere is 15.0 cm
Q50: A rock is suspended from a scale
Q57: X and Y are two uncharged metal
Q58: A thin,circular disk of radius 30.0 cm