Light shines through atomic hydrogen gas.It is seen that the gas absorbs light readily at a wavelength of 91.63 nm.What is the value of n of the level to which the hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level.(h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s, 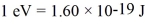 ,the Rydberg constant is R = 1.097 × 107 m-1)
,the Rydberg constant is R = 1.097 × 107 m-1)
Definitions:
Job Satisfaction Instrument
A tool or survey designed to measure the degree of contentment a worker feels towards their job.
Voluntary Turnover
Turnover initiated by employees (often when the organization would prefer to keep them).
Role Ambiguity
Uncertainty about what the organization expects from the employee in terms of what to do or how to do it.
Involuntary Turnover
Turnover initiated by an employer (often with employees who would prefer to stay).
Q6: Light shines through atomic hydrogen gas.It is
Q10: Light in air is initially traveling parallel
Q13: If the accuracy in measuring the position
Q29: A slit of width 2.0 μm is
Q30: The Fermi energy of rubidium at a
Q31: A nonrelativistic electron is confined to a
Q33: A muonic hydrogen atom is a proton
Q37: A coaxial cable consists of an inner
Q47: A three-dimensional potential well has potential U<sub>0
Q63: In the nuclear reaction<br>n + <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg"