How fast must a hydrogen atom be traveling for its kinetic energy to be just enough to excite the ground-state atom to its first excited state in a collision? (1 eV = 1.60 × 10-19 J, 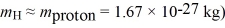
Definitions:
Exteroceptors
Sensory receptors that receive stimuli from the external environment, involved in senses such as sight, hearing, taste, smell, and touch.
Olfactory Cortex
A region of the brain involved in the processing of smell information received from the olfactory receptors.
Sensory Receptors
Specialized cells or nerve endings that respond to changes in the environment and send signals to the central nervous system.
Nasal Cavity
The large air-filled space behind the nose that filters, warms, and humidifies inhaled air.
Q5: A diatomic molecule is vibrating in its
Q17: Composite particles that are composed of a
Q19: A distant galaxy is emitting light from
Q20: Upon being struck by 240-nm photons,a metal
Q20: It is estimated that 3% of the
Q25: A giant star in another galaxy exploded
Q26: A random variable that can take on
Q27: A video rental store has two video
Q35: A manufacturer makes two products,doors and windows.Each
Q54: Trend in a time series must be