Multiple Choice
The normalized wave function for a hydrogen atom in the 1s state is given by 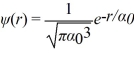 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
Understand the definition and context of a scientific theory and differentiate it from non-scientific usage.
Comprehend the evolutionary relationships and evidence supporting the ancestry of modern whales.
Identify the function of hemagglutinins in viruses and their role in the viral life cycle.
Recognize and explain homology and convergent evolution in the context of mammalian adaptations to aquatic life.
Definitions:
Related Questions
Q4: The risk neutral decision maker will have
Q6: How fast must a proton move so
Q18: If x is normally distributed with
Q20: If P(high)= .3,P(low)= .7,P(favorable | high)= .9,and
Q21: Frederick Taylor is credited with forming the
Q26: The expected utility is the utility of
Q36: The lifetime of an excited nuclear state
Q38: Management science and operations research both involve<br>A)qualitative
Q42: At absolute temperature T,a black body radiates
Q69: A convex lens has a focal length