How fast must a hydrogen atom be traveling for its kinetic energy to be just enough to excite the ground-state atom to its first excited state in a collision? (1 eV = 1.60 × 10-19 J, 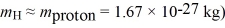
Definitions:
Implicit Attitudes
Unconscious beliefs or feelings towards people, objects, or concepts that can influence behavior and perception without conscious awareness.
Spontaneous Behavior
Actions that occur without premeditation or planning, often driven by innate impulses or reactions to immediate circumstances.
Self-Presentation Concerns
Worries or considerations regarding how one is perceived, judged, or evaluated by others.
Horror Movies
A genre of film intended to frighten, scare, disgust, or startle its audience by invoking their worst fears and nightmares.
Q4: What mass of <sup>14</sup>C (having a half-life
Q12: Today,the uranium found on Earth contains 0.720%
Q14: The magnitude of the Poynting vector of
Q19: A certain diatomic molecule emits a photon
Q20: Going from medium mass nuclei to heavy
Q25: An accounting firm has noticed that of
Q31: Utility reflects the decision maker's attitude toward<br>A)probability
Q34: The purchase of insurance and lottery tickets
Q41: A laser beam passes through a thin
Q44: In the lab,a relativistic proton has a