An ideal gas undergoes the process a→b→c→a shown in the pV diagram.In the figure,Pa = Pc = 240 kPa,Vb = Vc = 40 L,Va = 15 L,and Pb = 400 kPa.How much internal (thermal) energy is gained by the gas in this a→b→c→a process? 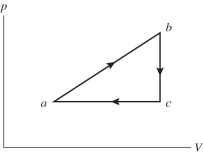
Definitions:
Pain Compliance
A law enforcement technique that uses pain to gain compliance from an individual without causing permanent injury.
Impact Techniques
involve strategies or methods designed to influence or change behaviors, attitudes, or perceptions in a targeted audience or system effectively.
Deadly Force Action
Utilizing force that can potentially cause death or serious injury to the body.
Domestic Violence
The abuse of one partner within an intimate or family relationship.
Q1: A child is riding a merry-go-round that
Q16: An air bubble underwater has the same
Q16: Consider a very small hole in
Q16: A small 0.050-kg insulating sphere carries
Q36: A temperature change of 20 C° corresponds
Q37: Consider a solid uniform sphere of radius
Q43: An object of 3.0-kg mass is located
Q61: Which one of the following is a
Q72: Which of the following changes will increase
Q129: Each plate of an ideal air-filled